The Pros Of Using A Battery Cover Even Though You Don’t Need One
You are installing a battery for your car, and you notice that the car has a battery cover and compartment. Do you need a car battery cover?
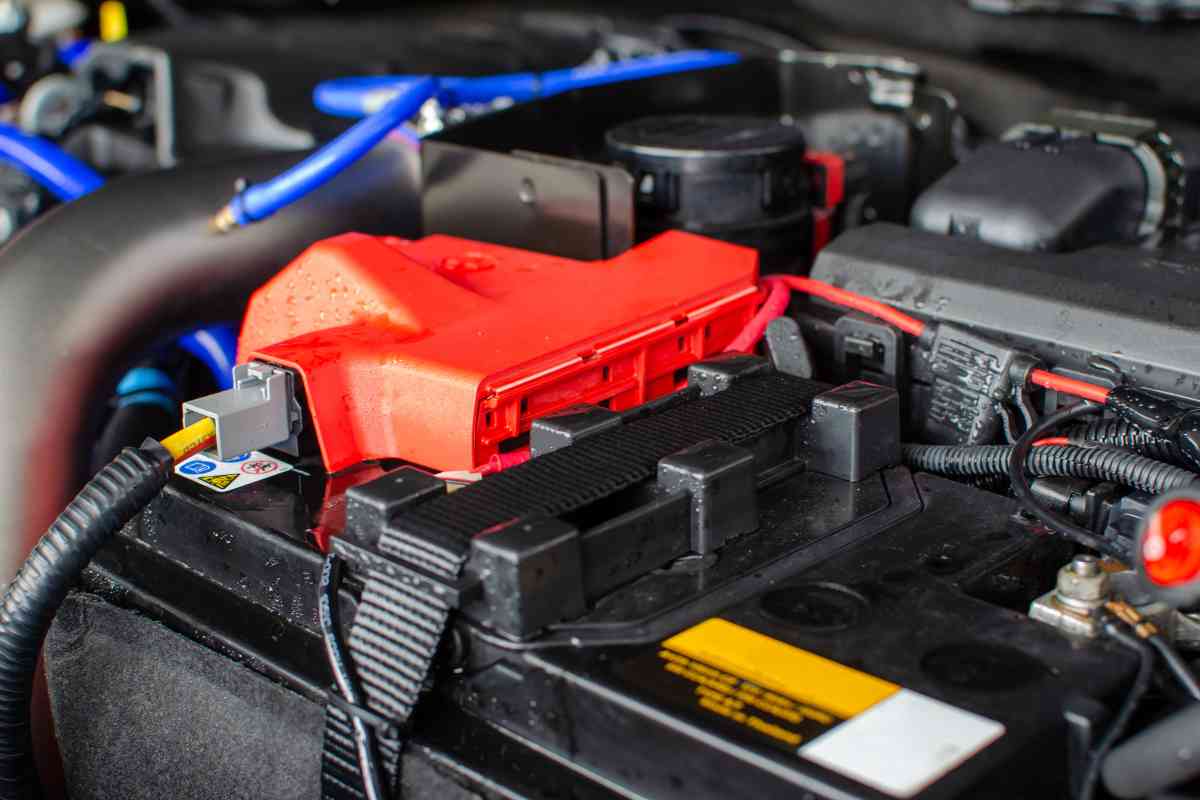
Do you need a battery cover?
You don’t need a battery cover, but they help protect the battery by separating it from the rest of the engine components as a safety measure. The cover keeps dirt and debris from settling on the battery, and plastic covers on the terminal posts can prevent corrosion from venting gases from most VLA batteries.
The auto manufacturer was not just trying to make your life more difficult when they created the housing for your battery or the plastic covers on top of the posts.
Even though it seems a no-brainer that the extensive red cover with a + on it is the positive terminal, there are other reasons for these plastic holders.
In this article, we will share some of the reasons why battery covers are important and why you should be glad your car is equipped with them.
What is a Battery Cover?
A battery cover is a plastic lid that works with the housing and brace bar to contain the battery and keep it separate from the rest of the engine components.
The construction of these covers is made of plastic, which does not conduct electricity, and prevents the battery from being short-circuited accidentally.
Why is a Battery Cover Important?

While the cover can keep a battery free of debris and dust or keep the terminals from coming in contact with each other, the primary reason for the cover and housing is for safety reasons.
The compartment for the battery can help keep a battery stationary in the event of a minor accident.
(In many cars, a brace over the middle of the battery connects with a bottom shelf to hold the unit firmly in place).
This housing prevents the battery from shifting loose or coming in contact with flammable materials (like gasoline or other fluids) and creating a fire that could cause extensive damage to the engine.
What do the Plastic Covers on the Battery Terminals do?
Just like the housing protects the box of the battery from damage, there are several reasons why a car manufacturer installs plastic covers on the posts.
Terminal Covers are Non-Conducive
The plastic of the covers prevents electrical current from short-circuiting the battery by accidentally connecting the two posts.
Because plastic does not conduct electricity, it is the perfect material to prevent you from getting shocked or the battery being fried because it came in contact with a foreign object like a tool or wrench.
Terminal Covers Remind us of Polarity.
On a battery with two posts, polarity is a big deal.
One post is positive, and the other is negative. If you have ever gotten the ends of a battery cable mixed up, you know what a disaster that situation can be (explosion, dead battery, injury, or engine fire).
The automaker makes one red and the other black to add another way to help us recognize what goes where.
Terminal Covers Reduce Exposure and Limit Corrosion
Terminal covers can help keep dust and debris off of the posts. In addition, they limit some of the exposure that contributes to the growth of corrosive elements.
While a neglected battery can still produce corrosion even with plastic covers, it takes longer to do so when they are applied.

Do I Have to Use a Battery Cover?
If you decide it’s too much of a hassle, you don’t have to put the cover back in place for the vehicle to run.
Many cars are more sophisticated, and many automakers have included a cover for a reason.
Tossing the battery cover aside will leave your battery more exposed to the elements, meaning a greater risk of corrosion and failure.
What if The Battery Cover is Cracked or Broken?
You need to decide whether you want to replace it or not. Most dealerships have extensive parts catalogs on their computers and can quote you the price for a replacement part.
Usually, the covers snap into place, so don’t think you need to have a technician do the work and share $100 for about 30 seconds of work.
How Does a Car Battery Work?
The most common type of battery found in cars and trucks is a VLA (Vented Lead Acid) or also known as a “flooded” or “wet” battery.
Inside the battery’s housing is a series of six cells composed of lead and lead dioxide plates resting in a sulphuric acid bath.
The acid acts as a catalyst to the plates producing electrical reactions which are channeled to your engine and other components through the cables connected to the terminal on top of the battery.
When the battery is charged, the electrolysis produces hydrogen and oxygen gases.
VLA batteries have small vents which act as pressure-release valves, keeping the hydrogen gases from building up and igniting so that your car doesn’t blow up or catch fire.
What is Corrosion?
When the hydrogen vapors connect with the heat generated by the engine, another chemical reaction occurs as they mix with other elements around them. This produces corrosion which often deposits itself on the hot battery terminals as a white, bluish, or green-colored substance.
(Since most battery connectors are copper, the substance produced is anhydrous copper sulfate.
Anhydrous means a crystalline structure. The corrosion turns blue or green with exposure to moisture).
This anhydrous copper sulfate is a nuisance for battery terminals. It can build up on the battery posts and the interior walls of the battery cables.
This condition means your battery cannot power everything in your car or recharge. This lack of charge means your battery underperforms).
Key Takeaways
- Battery covers can keep batteries clean and free of debris
- The covers also help keep a battery stationary in an accident.
- The covers can help prevent the corrosion of battery terminals.
- The terminal covers help remind us which post is positive and which is negative
- Corrosion is the result of a chemical reaction producing anhydrous copper sulfate
